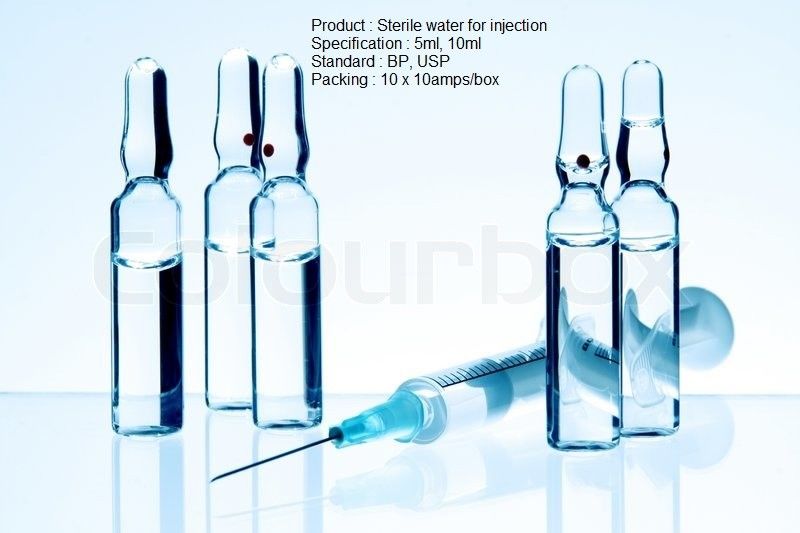
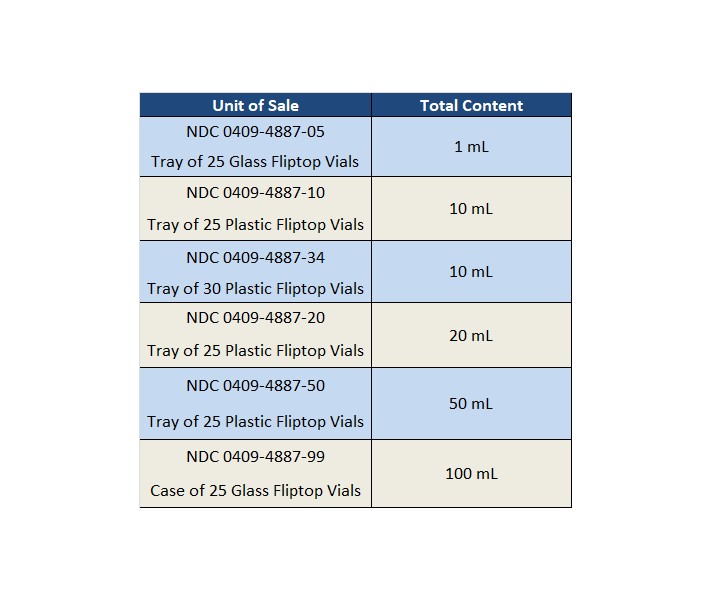
Sterile Water For Injection Usp Specification

It is intended to be used as a diluent in the preparation of parenteral products most typically for multi dose products that require repeated.
Sterile water for injection usp specification. Usp sterile water for irrigation the usp designation means that the water is the subject of an official monograph in the current us pharmacopeia with various specifications for each type. Great deals on chemicals and chemical supplies. Where the fill volume is 50 ml or more add 0 2 ml of 0 1 n potassium permanganate and boil for 5 minutes. For intravenous injection add sufficient solute to make an approximately isotonic solution.
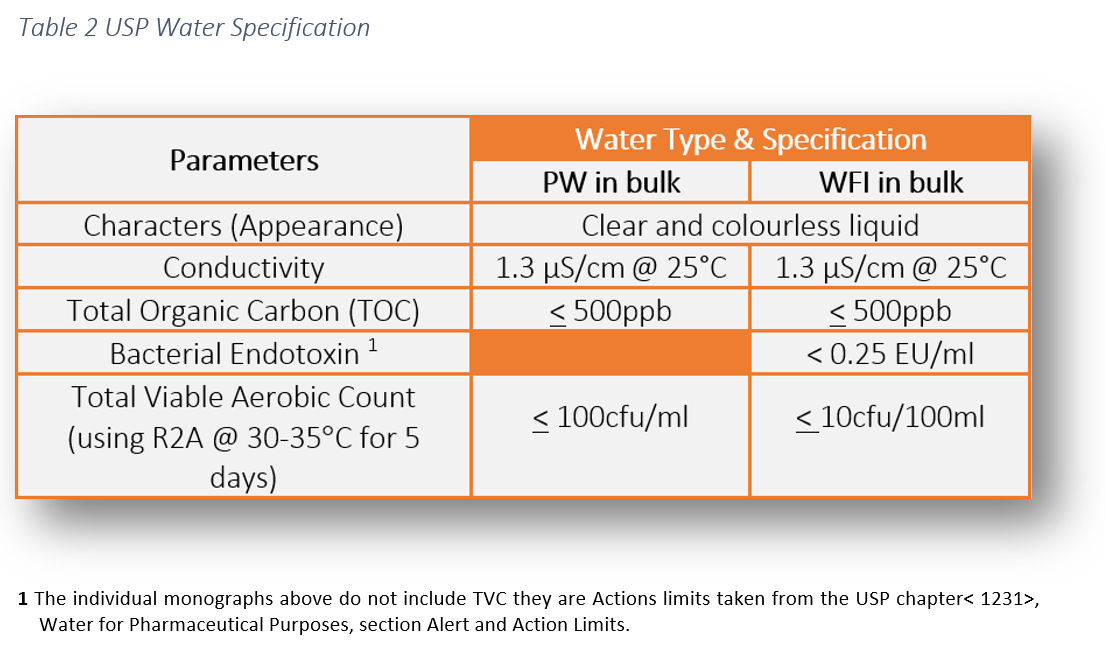
Sterile water for injection usp is a sterile nonpyrogenic preparation of water for injection which contains no bacteriostat antimicrobial agent or added buffer and is supplied only in single dose containers to dilute or dissolve drugs for injection. Usp standards for packaged purified water water for injection and sterile purified water usp24 effective 1 1 00 the following are numerical value limits that are commonly used interpretations of the procedures listed on pages 1752 and 1753 under the individual monographs. For sterile water for injection in containers having a fill volume of less than 50 ml add 0 4 ml of 0 1 n potassium permanganate and boil for 5 minutes. Note water for injection is intended for use in the preparation of parenteral solutions.
Bacteriostatic water for injection bacteriostatic water for injection see usp monograph is sterile water for injection to which has been added one or more suitable antimicrobial preservatives. Usp reference standards 11. Since purified water water for injection or the sterile waters are of such high purity the passage of time does not do anything except potentially degrade the sample due to environmental ambient or container factors. Allocation reset date.
Where used for the preparation of parenteral solutions subject to final sterilization use suitable means to minimize microbial growth or first render the water for injection sterile and thereafter protect it from microbial contamination. And 2 water is typically not produced in batches but rather it is usually purified produced and consumed continuously. Specification for water for injection wfi as per usp know the specification of water for injection wfi as per united states pharmacopoeia.